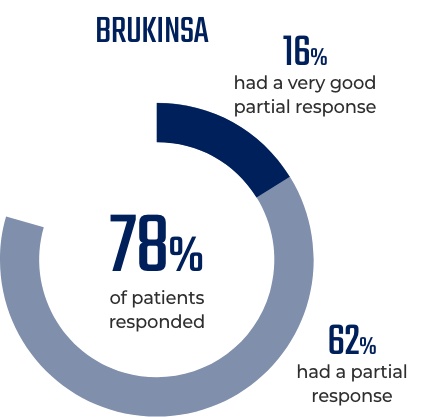
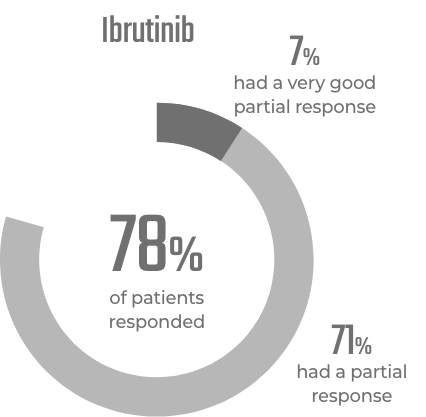
BRUKINSA is a BTK inhibitor that was designed to block BTK
- BRUKINSA has been shown to block 100% of BTK in blood cells and 94% to 100% of BTK in lymph nodes when taken at the recommended total daily dose of 320 mg. The significance of blocking up to 100% of BTK on treatment responses has not been established
Why is a BTK inhibitor important for
Waldenström’s macroglobulinemia (WM) treatment?
WM is caused by rapid growth and spread of cancerous B cells.
- Bruton’s tyrosine kinase (BTK) is a protein that signals within cancerous B cells, helping them to grow and spread
- Blocking BTK can help stop this signaling
The significance of blocking up to 100% of BTK on treatment responses has not been established.