BRUKINSA is a BTK inhibitor that was designed to block BTK
- BRUKINSA has been shown to block 100% of BTK in blood cells and 94% to 100% of BTK in lymph nodes when taken at the recommended total daily dose of 320 mg. The significance of blocking up to 100% of BTK on treatment responses has not been established
Why is a BTK inhibitor important for CLL/SLL treatment?
- Bruton’s tyrosine kinase (BTK) is a protein that signals within cancerous B cells (like those that cause CLL or SLL), helping them to grow and spread
- Blocking BTK can help stop this signaling
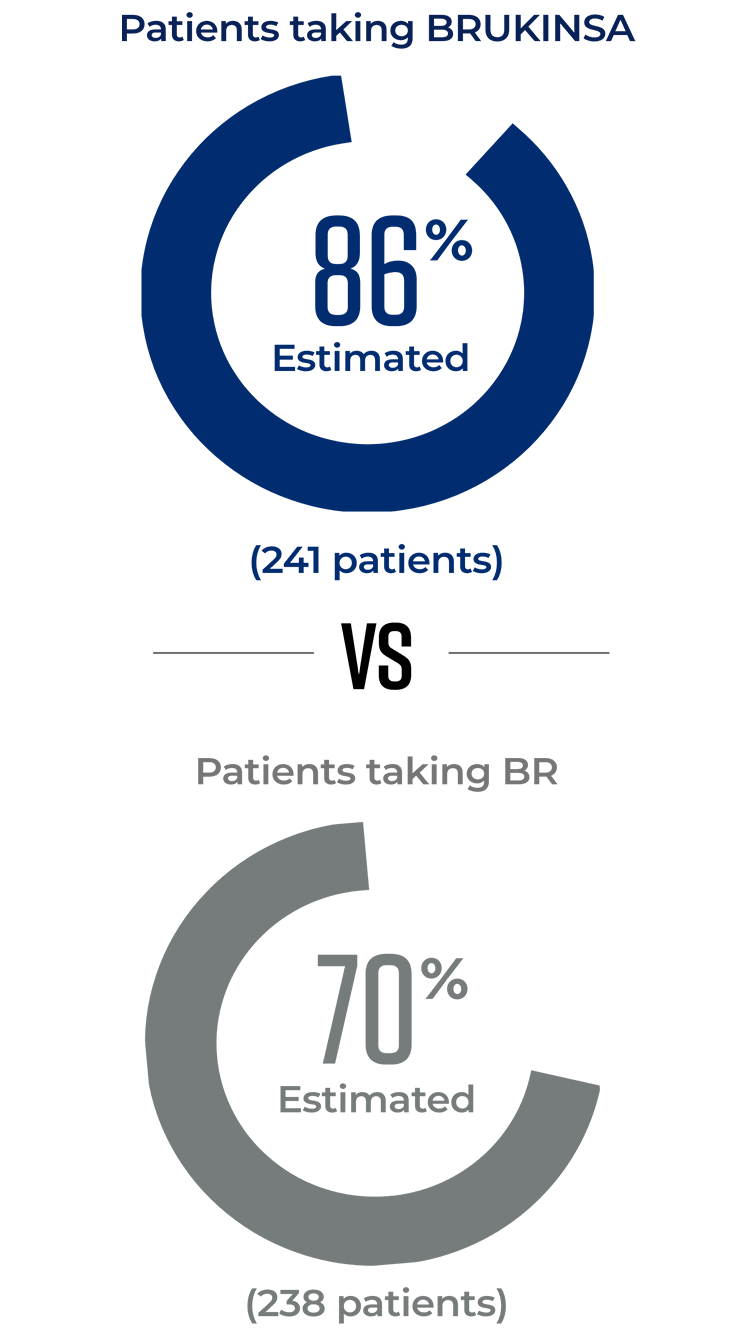
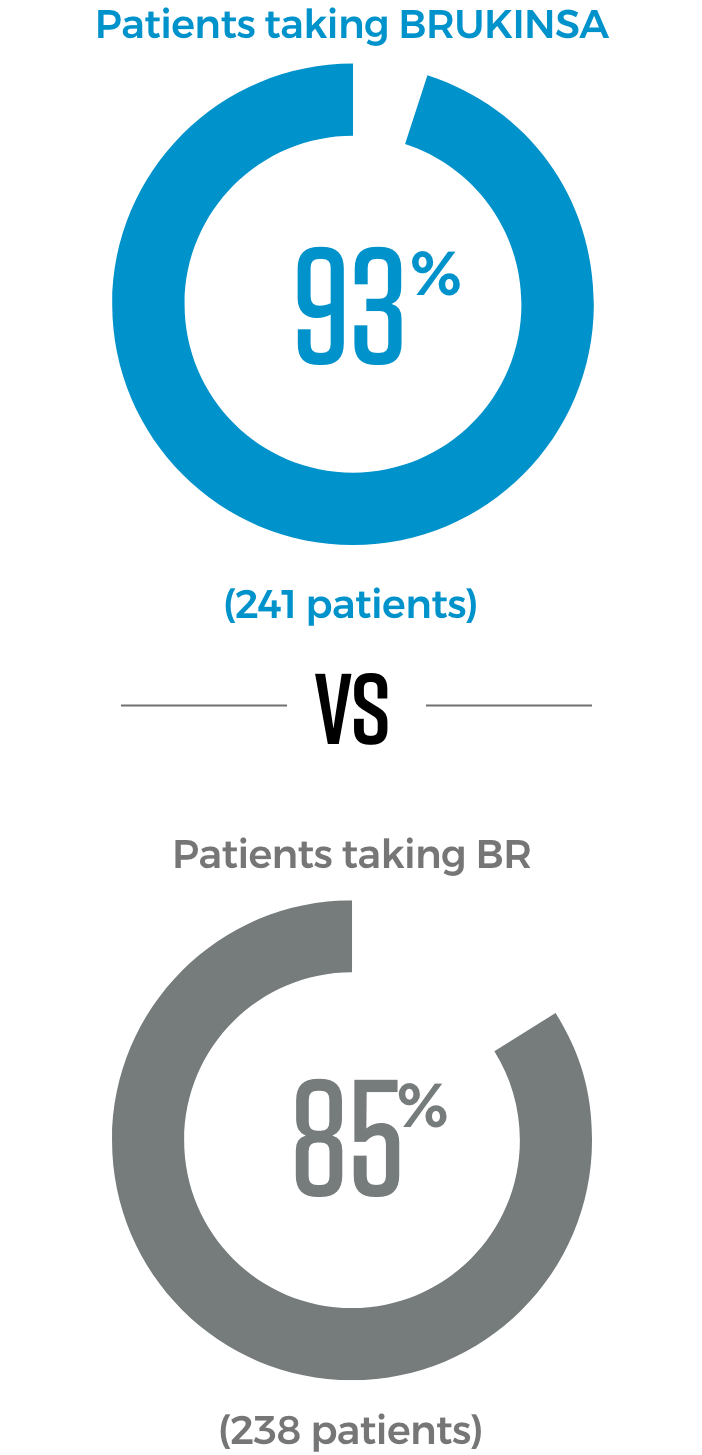
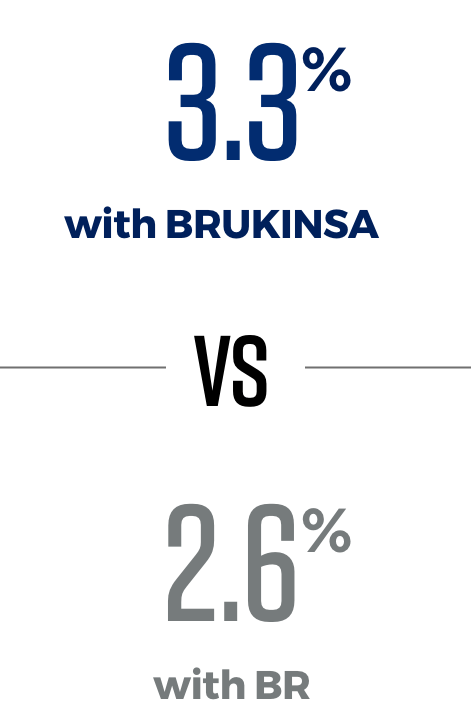
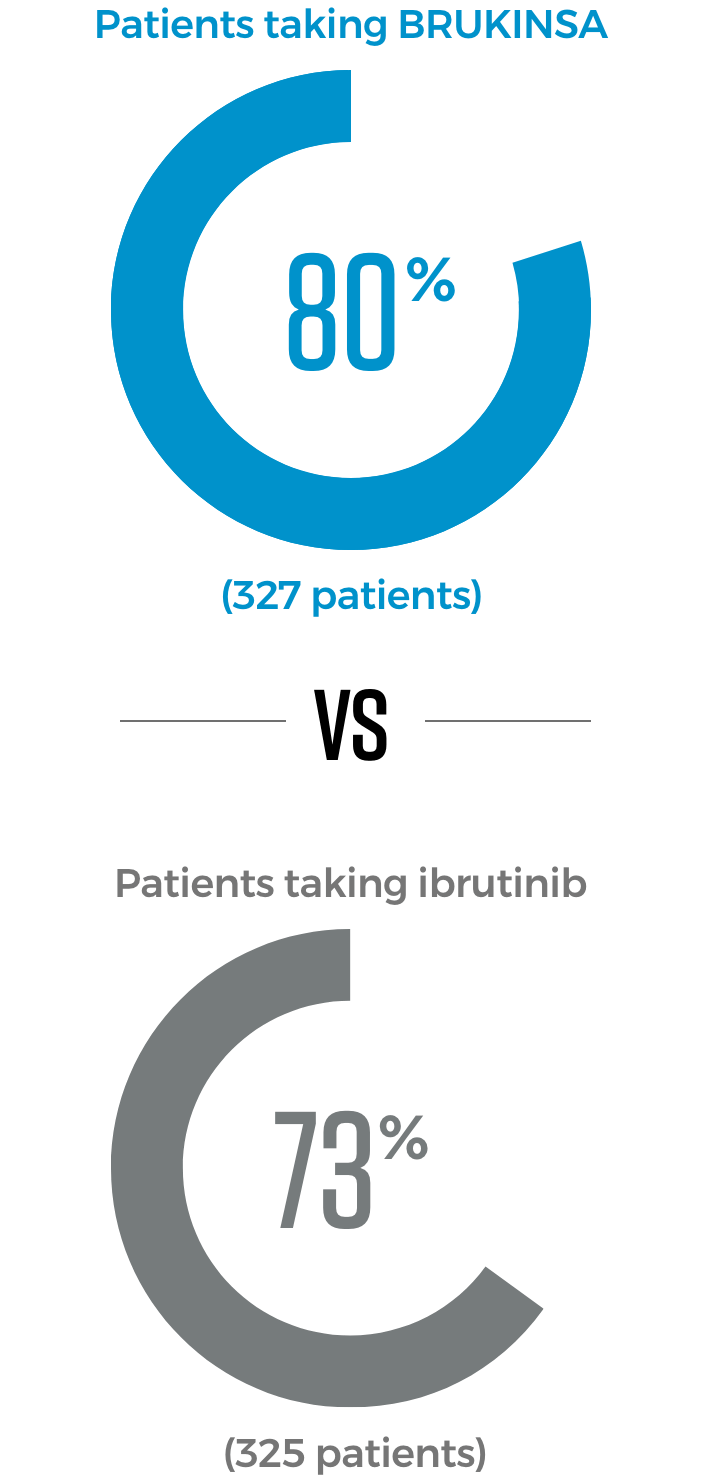
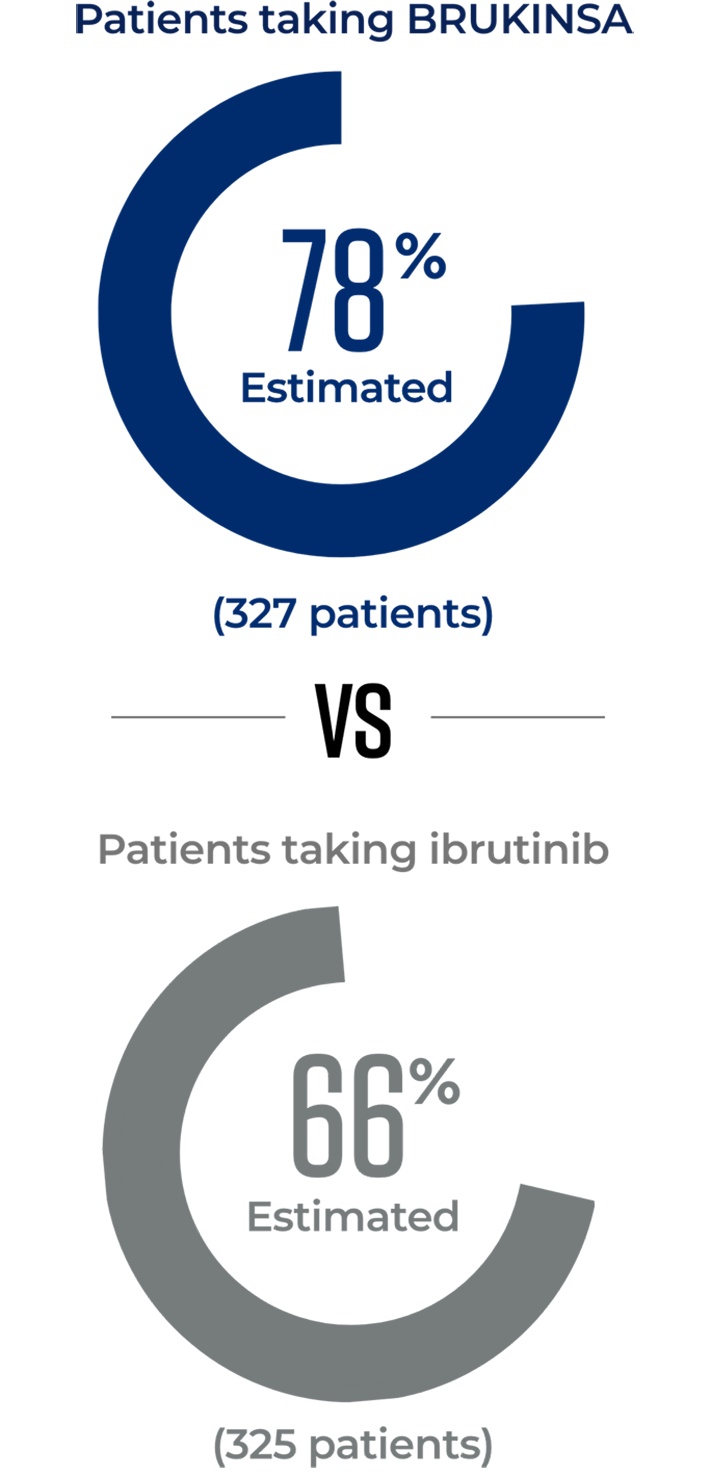
In 2 trials directly comparing BRUKINSA to other approved CLL/SLL treatment options, significantly more patients taking BRUKINSA achieved better results
BRUKINSA is the only BTKi proven to be superior to both another BTKi and to chemotherapy